|
Copyright ©2011 by Paul Niquette. All rights reserved. |
|||
Fill a glass with ice cubes and enough water to bring the level to the brim and ask, "Will the table stay dry?" The floating ice will actually be seen above the brim.
Published by permission of the artist.
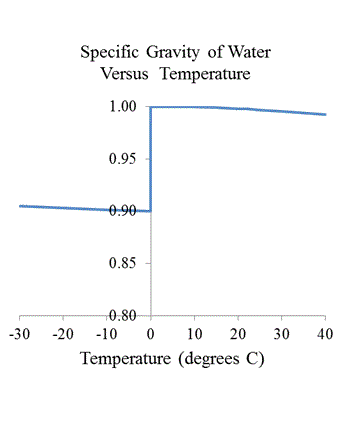
That moonless night of 14 April 1912 when the mighty RMS Titanic with 2,223 souls on board collided 37 seconds after the sighting of a particular iceberg and the fateful announcement by lookouts, "Iceberg right ahead!"There can be no doubt that what makes "Only the tip of the iceberg" work so well as a metaphor is the universal knowledge of a singular property of frozen water: It floats on water. Everybody also knows that water is that most abundant substance on the planet, which covers nearly 71% of the surface and constitutes up to 78% of our bodies.No wonder that water has been appropriated as a scientific standard. For example, in characterizing density, scientists use the expression specific gravity (SG), which is the ratio of the mass of a given body's volume to the mass of a reference body having an equal volume. The reference body is -- well, water. Accordingly, it is kind of ironic to study the SG of water itself, as shown in this little graph...
...in which solvers are invited to make the following observations about the SG of water:
 ew substances
expand like water when they freeze, specifically certain
crystal-forming compounds and the following chemical
elements: antimony, bismuth, gallium, germanium,
silicon, and plutonium. One wonders: To what
extent does life as we know it here on the planet earth
depend on this particular property of H2O? ew substances
expand like water when they freeze, specifically certain
crystal-forming compounds and the following chemical
elements: antimony, bismuth, gallium, germanium,
silicon, and plutonium. One wonders: To what
extent does life as we know it here on the planet earth
depend on this particular property of H2O?
Our Tip of the Ice Cube puzzle is offered here in the form of a simple thought experiment that pertains to the theoretical ability of a lookout on board a vessel to see the tip of the iceberg far enough away to avoid collision with it 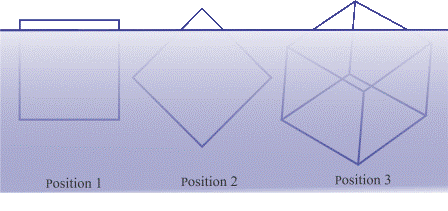
|